OncoBone has partnered with a small-molecule CDMO service provider to expand its drug development service offering. Below you can find more information about the services, and you can request additional information by reaching info@oncobone.com.
Scope of the CDMO offering
The partner CDMO of OncoBone has been a trusted provider since 2001. They operate in 3 000 m2 modern facilities including an R&D laboratory, GMP pilot manufacturing units, final handling and analytical laboratories. They are specialized in process chemical R&D services and cGMP manufacturing of small-molecule Active Pharmaceutical Ingredients (APIs) for early-stage drug development including preclinical and phase 1 and 2 clinical studies.
Key service areas of the partner CDMO are described below in Figure 1. They operate both with new chemical entities (NCEs) and generic market compounds according to current Good Manufacturing Practice (cGMP) standards. Their facilities are inspected regularly by the European Medicines Agency (EMA/FIMEA) and the U.S. Food and Drug Administration (FDA) and by their partners and customers.
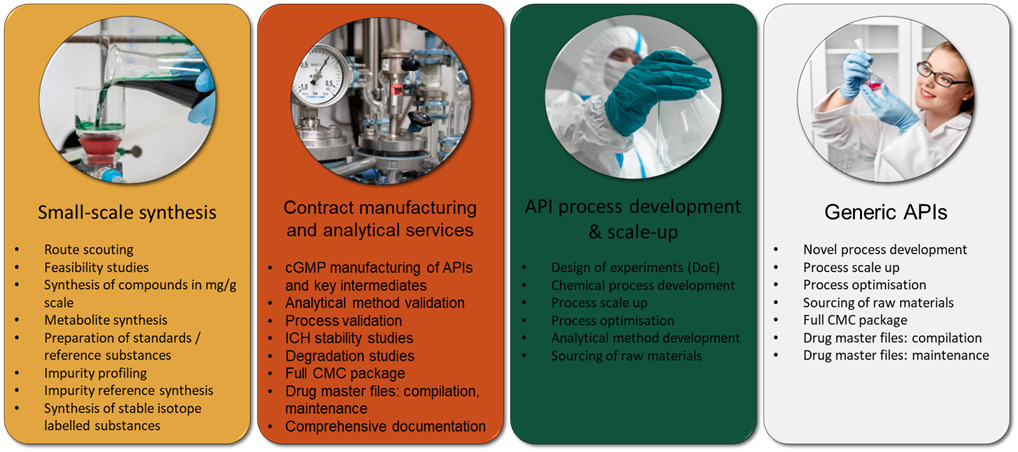
Figure 1: Summary of the small-molecule CDMO service offering
Summary of key expertise areas
- Broad expertise in organic chemistry
- Experienced personnel for resolving complex chemical problems
- Process R&D, scale-up and optimization
- Routinely inspected by regulatory authorities and customers
- Last EMA audit in 2021, next FDA audit to be performed in 2023
- Customer audits annually
- Small, flexible company with highly motivated personnel
- Focus on quality and timely delivery
- Open personal level communications
If you are interested to learn more, please contact us at info@oncobone.com.


